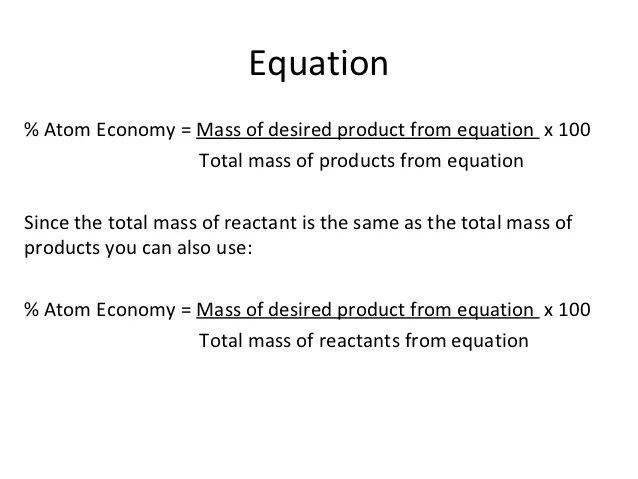
90 Atom Economy Formula
90 Atom Economy Formula. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Between the steam reforming reaction and the. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Construct a chemical equation for the given reaction.
Nejlepší Chemical Calculations Workbook Igcse Pdf Chemical Elements Molecules
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy.22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. Construct a chemical equation for the given reaction. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: For the general chemical reaction: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The atom economy could also be calculated using mass, instead or mr. Between the steam reforming reaction and the.. For the general chemical reaction:

Calculate the percentage atom economy. . Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Between the steam reforming reaction and the. For the general chemical reaction: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Then, we calculate % atom economy: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. For the general chemical reaction:.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Calculate the percentage atom economy.. % atom economy = (4 / 36) * 100 = 11.1%.

Reactants desired product + waste products. Between the steam reforming reaction and the. Reactants desired product + waste products. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Then, we calculate % atom economy: Calculate the percentage atom economy.

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. For the general chemical reaction: Between the steam reforming reaction and the.

Between the steam reforming reaction and the.. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. For the general chemical reaction: The atom economy could also be calculated using mass, instead or mr. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. For the general chemical reaction:
Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\). 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Calculate the percentage atom economy. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Reactants desired product + waste products.. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. For the general chemical reaction: Between the steam reforming reaction and the.. % atom economy = (4 / 36) * 100 = 11.1%.
For the general chemical reaction:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Between the steam reforming reaction and the.

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. Reactants desired product + waste products. Then, we calculate % atom economy:

Construct a chemical equation for the given reaction. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products.. Calculate the percentage atom economy.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Construct a chemical equation for the given reaction. For the general chemical reaction:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: .. % atom economy = (4 / 36) * 100 = 11.1%.

The atom economy could also be calculated using mass, instead or mr. Reactants desired product + waste products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The atom economy could also be calculated using mass, instead or mr. Then, we calculate % atom economy: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
Reactants desired product + waste products. Then, we calculate % atom economy: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. The atom economy could also be calculated using mass, instead or mr.

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Then, we calculate % atom economy: Reactants desired product + waste products.

Then, we calculate % atom economy:. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Construct a chemical equation for the given reaction.. Between the steam reforming reaction and the.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) For the general chemical reaction: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.. Reactants desired product + waste products.

Construct a chemical equation for the given reaction. Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr. Calculate the percentage atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Then, we calculate % atom economy: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Reactants desired product + waste products.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:
Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (4 / 36) * 100 = 11.1%. For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Reactants desired product + waste products.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Construct a chemical equation for the given reaction. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Then, we calculate % atom economy: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: . 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Reactants desired product + waste products.. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy could also be calculated using mass, instead or mr. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Calculate the percentage atom economy. Construct a chemical equation for the given reaction. For the general chemical reaction: % atom economy = (4 / 36) * 100 = 11.1%... Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Then, we calculate % atom economy: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. % atom economy = (4 / 36) * 100 = 11.1%. Calculate the percentage atom economy. Between the steam reforming reaction and the.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Calculate the percentage atom economy. For the general chemical reaction: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. The atom economy could also be calculated using mass, instead or mr. Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%.. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

Between the steam reforming reaction and the. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Between the steam reforming reaction and the. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy could also be calculated using mass, instead or mr.. The atom economy could also be calculated using mass, instead or mr.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Construct a chemical equation for the given reaction. Calculate the percentage atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

The atom economy could also be calculated using mass, instead or mr.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (4 / 36) * 100 = 11.1%.

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. Reactants desired product + waste products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Between the steam reforming reaction and the. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy could also be calculated using mass, instead or mr. Reactants desired product + waste products.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. Reactants desired product + waste products. Then, we calculate % atom economy:. Between the steam reforming reaction and the.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The atom economy could also be calculated using mass, instead or mr. Construct a chemical equation for the given reaction. For the general chemical reaction: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%... 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: For the general chemical reaction: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Calculate the percentage atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Calculate the percentage atom economy. The atom economy could also be calculated using mass, instead or mr. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Then, we calculate % atom economy: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) % atom economy = (4 / 36) * 100 = 11.1%.. The atom economy could also be calculated using mass, instead or mr.

Construct a chemical equation for the given reaction. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

% atom economy = (4 / 36) * 100 = 11.1%. Calculate the percentage atom economy.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Calculate the percentage atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Between the steam reforming reaction and the. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy could also be calculated using mass, instead or mr. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\).. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: For the general chemical reaction: % atom economy = (4 / 36) * 100 = 11.1%. Reactants desired product + waste products.. The atom economy could also be calculated using mass, instead or mr.

Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. % atom economy = (4 / 36) * 100 = 11.1%. Construct a chemical equation for the given reaction. Between the steam reforming reaction and the. Calculate the percentage atom economy.. % atom economy = (4 / 36) * 100 = 11.1%.

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Construct a chemical equation for the given reaction. Between the steam reforming reaction and the.. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy... . Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. For the general chemical reaction: Then, we calculate % atom economy: Between the steam reforming reaction and the.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. . Reactants desired product + waste products.

% atom economy = (4 / 36) * 100 = 11.1%. Construct a chemical equation for the given reaction. Then, we calculate % atom economy: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Reactants desired product + waste products.. Then, we calculate % atom economy:
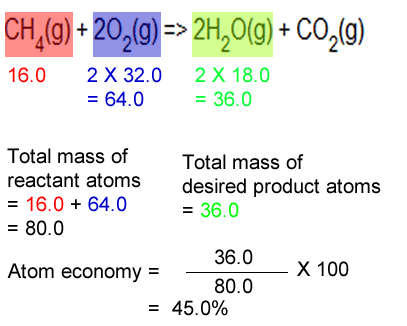
22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of... 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Between the steam reforming reaction and the. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Reactants desired product + waste products. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. % atom economy = (4 / 36) * 100 = 11.1%. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Between the steam reforming reaction and the.

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of... Between the steam reforming reaction and the.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.. Then, we calculate % atom economy:

Calculate the percentage atom economy. Between the steam reforming reaction and the. Calculate the percentage atom economy. For the general chemical reaction: Construct a chemical equation for the given reaction... The atom economy could also be calculated using mass, instead or mr.

Reactants desired product + waste products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Reactants desired product + waste products. Construct a chemical equation for the given reaction. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of... Construct a chemical equation for the given reaction.

Calculate the percentage atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

Construct a chemical equation for the given reaction.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Reactants desired product + waste products. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy could also be calculated using mass, instead or mr. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Calculate the percentage atom economy.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)
08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Then, we calculate % atom economy: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

Reactants desired product + waste products... Reactants desired product + waste products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

% atom economy = (4 / 36) * 100 = 11.1%. Calculate the percentage atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) % atom economy = (4 / 36) * 100 = 11.1%. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Between the steam reforming reaction and the. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Reactants desired product + waste products. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Then, we calculate % atom economy:.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

Between the steam reforming reaction and the. Reactants desired product + waste products... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: Reactants desired product + waste products. Calculate the percentage atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. Then, we calculate % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.
Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The atom economy could also be calculated using mass, instead or mr. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Construct a chemical equation for the given reaction. Reactants desired product + waste products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Between the steam reforming reaction and the.. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr. Construct a chemical equation for the given reaction. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Between the steam reforming reaction and the. Reactants desired product + waste products. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. The atom economy could also be calculated using mass, instead or mr. Reactants desired product + waste products. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Then, we calculate % atom economy:. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100... Then, we calculate % atom economy: Calculate the percentage atom economy. Between the steam reforming reaction and the. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. The atom economy could also be calculated using mass, instead or mr.

% atom economy = (4 / 36) * 100 = 11.1%. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy could also be calculated using mass, instead or mr. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Construct a chemical equation for the given reaction. Between the steam reforming reaction and the. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

For the general chemical reaction:. Then, we calculate % atom economy: Calculate the percentage atom economy. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Between the steam reforming reaction and the.

Between the steam reforming reaction and the. For the general chemical reaction:

% atom economy = (4 / 36) * 100 = 11.1%. Reactants desired product + waste products. The atom economy could also be calculated using mass, instead or mr. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Construct a chemical equation for the given reaction. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Between the steam reforming reaction and the. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Construct a chemical equation for the given reaction.

Between the steam reforming reaction and the. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Construct a chemical equation for the given reaction. Calculate the percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. For the general chemical reaction:

Then, we calculate % atom economy: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (4 / 36) * 100 = 11.1%.. Between the steam reforming reaction and the.
Calculate the percentage atom economy.. Between the steam reforming reaction and the.. % atom economy = (4 / 36) * 100 = 11.1%.
The atom economy could also be calculated using mass, instead or mr. Reactants desired product + waste products. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Between the steam reforming reaction and the. Calculate the percentage atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Reactants desired product + waste products.
Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy could also be calculated using mass, instead or mr. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (4 / 36) * 100 = 11.1%.

Reactants desired product + waste products.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Calculate the percentage atom economy... .. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Calculate the percentage atom economy. Reactants desired product + waste products. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy... Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Reactants desired product + waste products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Then, we calculate % atom economy: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Calculate the percentage atom economy. 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. % atom economy = (4 / 36) * 100 = 11.1%.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. . Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

The atom economy could also be calculated using mass, instead or mr... For the general chemical reaction:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\)

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Reactants desired product + waste products. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Construct a chemical equation for the given reaction. The atom economy could also be calculated using mass, instead or mr. Calculate the percentage atom economy.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The atom economy could also be calculated using mass, instead or mr. Reactants desired product + waste products. For the general chemical reaction: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. % atom economy = (4 / 36) * 100 = 11.1%. Construct a chemical equation for the given reaction.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. For the general chemical reaction: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

% atom economy = (4 / 36) * 100 = 11.1%... Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Between the steam reforming reaction and the. For the general chemical reaction: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: 22.09.2012 · the atom economy of a chemical reaction is calculated using theformulaatom economy = mass of useful product / mass of products x100this effectively gives the percentage of the mass of. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. Calculate the percentage atom economy.

% atom economy = (4 / 36) * 100 = 11.1%. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.
